Lindenbaum ES, PhD, Feitelberg AL, MD, Tendler M, PhD, Beach D, MSc, Gamliel-Lazarovich A, MSc, Har-Shai Y, MD, Hirshowitz B, MD
Dermatol Online J 9(1), 2003. © 2003 Arthur C. Huntley, MD
Androgenetic Alopecia (AA) afflicts a large part of the population and of the many treatments available today none is completely satisfactory. Testing the efficacy and safety of a novel topical treatment for AA which is based on cell culture medium supplemented with insulin, thyroxin and growth hormone (CCM). The 48 participants classified as androgenetic alopecia Type II, III or IV on the Hamilton scale, concluded a randomized, vehicle-controlled, double-blind trial of 6 months duration. Under occlusive cover the gel was self applied for at least 3 hours daily. Evaluation was based on hair counts, investigator global assessment and participants self-administered questionnaire. Cessation of hair loss was reported by most participants within 2?8 weeks, and further confirmed by the hair count (HC) in ~80% of participants. Moreover, as early as 4 months after the start of the treatment, a time dependent increase of up to 50% in HC was observed. The average change in HC between the two groups differed significantly (p=0.007), with values of 4.1% for control and 13.8% for CCM. Following 4 months of treatment, a time dependent increase in HC (>10%) above minimal was observed in 55% of the CCM and 25% of the control and this trend continued. At 6 months 63% of the CCM and 33% of the control group exhibited increase of HC higher than 10%. The average increase in HC in the CCM and the control groups was 17.1% and 8.9% respectively (p=0.035). Self evaluation questionnaires revealed a time dependent increase in satisfaction in the CCMusers compared to the control. While the average score at T2was similar in CCM and control (2.7 and 2.6 respectively), the score at T6in the CCM increased to 5.9 and decreased to -0.4 in the control (p=0.007). Global-clinical evaluation following six months treatment revealed significantly (p=0.02) more hair loss in the control group (40%) compared to the CCM (7%) treated group. CCM was found effective in treating androgenetic alopecia in men. It induced cessation of hair loss, increased rate of hair growth and appearance of new hair. No side effects were reported or observed.
Introduzione:
Male pattern baldness, to its various degrees, afflicts a large part of population, starting as early as the late teens and increases with age.[1] The reduced scalp hair cover and regression of hair line are the result of continous degeneration of hair follicles (HF). Hereditary and endocrine factors are known to affect this process in ways which are not completely understood. Among the factors which are known to modulate hair growth are those that stimulate the circulation in the capillary plexus of the hair papilla and surrounding the hair sheath.[2] Increased vascular supply of nutrients and growth factors is required for vitalization of HF and synthesis of keratin to form the hair shaft. The mechanism of action of drugs that improved the vascular supply, such as the peripheral vasodilator Minoxidil, may be responsible for the increased hair growth.
In previous clinical studies,[3] healing of wounds with undermined blood supply was accelerated when cell culture medium supplemented with non-steroidal anabolic hormones (CCM) was topically applied. Not only were the wound bed cells revitalized by the direct supply of nutrients and hormones but it induced vascular granulation tissue formation accompanied by re-epithelialization and growth of hair in the skin adjacent to the wound.
Several types of media supplemented with hormones and growth factors were previously reported to support HF cells and organ cultures.[4, 5, 6, 7] In vivo studies[8] showed faster hair regrowth in chemotherapy induced alopecia in mice topically treated with CCM. Furthermore, a clinical pilot study on 15 participants using topical CCM once a day yielded cessation of hair loss, increased rate of hair elongation and growth of new hair.
Based on the above, a vehicle-controlled, randomized, double-blind study was designed to test the hypothesis that CCM will revitalize hair follicles in androgenetic alopecia in men.
Materials and Methods
This study was approved by the Institutional Review Board (Helsinki Committee) of the Ha’Emek Hospital and by the Israel Ministry of Health. All participants signed an informed consent form.
Participants:
After screening, 56 young men aged 18-39 years were admitted to the study, of which 48 completed the full 6 months duration. Inclusion criteria stipulated good physical and mental health, dark hair color, progressive hair loss with diffuse vertex alopecia at stage II, III or IV on the Hamilton scale. Participants were randomly assigned to either CCM or to the control group. For the period of the clinical trial, usage of any hair product such as hair rinses, dyes, conditioners, sprays, setting gels, restorers and shampoos except the recommended baby shampoo was discontinued as were any procedures for hair enhancement and curling or straightening techniques.
Each participant visited the laboratory once a month for examination, data collection, submission of empty containers of the gel and the treatment diary of the previous month and for supply of CCM for the next month treatment.
Cell Culture Medium:
The MCDB153 (CCM) (Biological Industries, Israel) was supplemented with three non-steroidal anabolic hormones, insulin (Novo Nordisk, Denmark), thyroxin (Knoll Pharmaceuticals, USA) and growth hormone (Biotechnology General, Israel) gelled in 1.5% hydroxyethylcellulose (Hercules, Netherlands). The control was gelled saline. The outward appearance of both control and active gels was identical.
Applicazione:
Participants were given oral and written instructions on the use of the gel. A daily topical application of 1-3 ml of gel to a hydrated scalp skin was recommended. The head was then covered with plastic occlusive cap for a period of at least 3hrs followed by an additional 3 hours with or without scalp cover. Most participants preferred an overnight treatment. The gel which is readily soluble in water was rinsed out. Each participant kept records of the length of time the occlusive cover was in place and the total length of treatment.
Microfotografia:
Standardized 10x magnified photographs (Dermaphot, Heine, Germany) were taken at the start of the experiment (T0) and then, after 2, 4 and 6 months (T2, T4, T6). Sites chosen were at the peripheral zone of the bald area where 30% hair loss was estimated. Either a natural mole (hemangioma) or a tattooed dot were used to mark the center of the site selected for photography. The hair around the dot was clipped and photographs were taken with and without immersion oil.
Hair Count:
Hair counts (HC) were performed on microphotographs. All the hair, in an 0.5cm2 area (0.8cm2) surrounding the designated mole or tattooed dot, was counted by two independent investigators. Each photograph was compared to previous photographs by at least 4 ‘flags’ to confirm that the sites were identical. The change in HC was normalized to the initial HC at T0 using Ti– T0/T0 (where Ti represent HC at 2, 4 or 6 months), and the results were expressed as % of increase in HC. Reproducibility of counts in a random sample of microphotographs indicated accuracy regardless of counting experience. The data collected was statistically analyzed using chi-square test.
Investigators global evaluation
Standardized macrophotographs (Minolta 300s) were taken for global evaluation. The head of each participant was always placed in a prefixed identical position and the photograph was taken from the same distance and angle. For standardization the participants were groomed according to the style and cut they had in the previous photographs and a blue background screen was used.. Global evaluation was blindly assessed by two independent clinicians (a dermatologist and a plastic surgeon) by comparing the T0 photograph with T2, T4 and T6 photographs. The score of the visual changes in the appearance of the hair ranged from -2 to +2 where no change scored zero.
Patients Evaluation (Self-evaluation questionnaire):
At T2, T4 and T6 the participants, blinded to their treatment, answered a scaled questionnaire evaluating their satisfaction with the treatment. The questions focused on general well being, the effectiveness of the treatment in slowing the hair loss, on manifestations of any discomfort or symptom of skin or other irritations, satisfaction with the hair growth rate and on the appearance of hair in general and in the bald area in particular. Each answer referred to the change from the start of the treatment. Scores are in the range of -3 to +3 where no change scored zero. The data was calculated both as the average sum of the scores of each participant in a treatment group and also on a segmentation score of the positive replies of each participant.
Results
Of the 56 volunteers enrolled in this experiment, 48 completed the 6 months study and 8 non compliant participants were discontinued. There were 30 participants in the CCM treated group and 18 in the vehicle control group. Data was collected over a period of two years. The initial demographic data was similar in the control and the CCM treated groups and is summarized in Table I.
Hair Count:
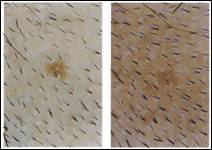
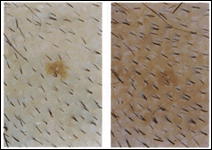
Microphotographs of the scalp reveal increase in hair density and change in the prevalent type of hair. While at the start of the treatment a large portion of the hair was vellus to intermediate type and devoid of pigment, at the end of the treatment much of the hair became intermediate to terminal type and pigmented (Fig 1). In this study, the hair counts included all types of hair.
 |
 |
A sinistra: Microphotographs of CCM treated participants at T0 (a) and at T6 (b).Note the difference in hair density. Some of the existing which were vellous and intermediate type at the beginning of the treatment, became terminal and pigmented. The increase in HC from (a) to (b) was 50%
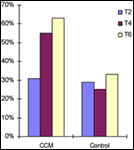
A destra: Level of change in HC – A time dependent segmentation of an above minimal change in HC (>10%) within treatment groups. Source: medscape.com |
For each participant, the initial HC served as a baseline to calculate the change following treatment. The results are summarized in Table II. After two months of treatment, similar average increase in HC was observed in both CCM and control. However, CCM treatment yielded a further time-dependent significant increase in HC which was not observed in the control. After 6 months, the increase in HC following CCM treatment reached a value which is twice that measured in the control.
The increase in number of hair may not reflect the degree of beneficial results of treatment since variation in hair density between individuals tend to be high. Therefore, the normalized change in HC i.e., the ratio of change in HC to the baseline, is a better indicator of treatment efficacy. An increase of up to 50% in HC was found in the CCM treated group as early as 4 months, with an average of 13.8% compared to 4.1% in the control (Table III). A further increase was demonstrated following 6 months where average increase in HC was 8.9% and 17.1% in the control and CCM groups respectively .
Not only was the average percent of change in HC significantly higher in the CCM group compared to the control but segmented level of increase resulted in even more remarkable findings. Minimal increase in HC was defined as percentage of change higher than 10%. The distribution of the above minimal increase within the CCM and the control is shown (Fig 2). After 2 months of treatment, 30% of participants in both CCM and control groups showed an above minimal increase in HC. However, the segmental distribution showed a definite time-dependent deviation between the two groups. After 6 months of treatment, there were twice as many participants in the CCM group exhibiting an above minimal increase in HC compared to the control (63% compared to 33%) .
Investigators global evaluation:
Investigators evaluation of macrophotographs comprised changes in hair density and size of bald area. In macrophotographs which revealed increase in hair density, the increase was mostly at the perimeter of the bald area. Therefore in these cases the size of the bald area was reduced
 |
 |
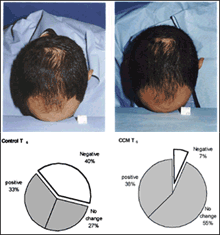
A sinistra: Sopra, Macrophotographs of global hair appearance in CCM treated participant at T0 (a) and at T6 (b). After 6 months of treatment the density of the hair increased and the perimeter of the bald area was reduced. Global evaluation score from (a) to (b) was +1. Sotto, Investigator global evaluation
A destra: Self evaluation indicating the participants satisfaction with the treatment Source: medscape.com |
Efficacy of treatment in terms of global appearance evaluated by independent investigators did not agree with the HC results within the time scope of this study. Still, a trend of improvement in the CCM treatment group and worsening in the control can seen, and cosmetic changes became evident following 6 months of treatment. When evaluation of global changes was simplified to the following three categories: no change, positive or negative change, a significant deviation between the two treatments was observed. For 40% of the control group, the hair appearance worsened after 6 months of treatment, and only 7% in the CCM group showed similar change (p=0.02). The extent of improvement was similar in both groups.
Participants evaluation:
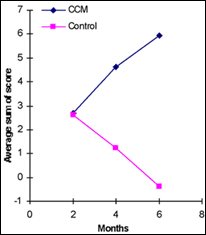
Participants satisfaction with the results of treatment was revealed by the responses to a scaled questionnaire. Each question focused on one parameter of hair appearance and the response measured the nature and amplitude of change from the start of the experiment. Thus, the sum of scores of the 8 questions obtained from each participant indicates the level of satisfaction with the results of treatment. The mean values revealed that the group treated with CCM was significantly more satisfied with the treatment results than the control group in a time-dependent manner Moreover, an opposite perception of treatment efficacy was found, with growing dissatisfaction in the control treatment group and an increased contentment in the CCM group. After 6 months, the percentage of participants with sum of scores greater than zero varied significantly (p=0.007) between CCM and control (79% and 38% respectively).
In addition, most of the CCM treated volunteers reported a reduction in scalp dryness and dandruff, an improvement in the “texture and strength” as manifested by the hair remaining attached to the scalp after pulling on it, and a noticeable enhancement of hair growth rate.
Compliance:
Comparison of saline and CCM daily records of treatment revealed similarity. Within the 6 months trial, the average number of daily treatments was 150 and the average length of each application was 4.6 hours/day. No correlation was found between duration of treatment and change of hair counts or global evaluation. Furthermore, low scores of self-evaluation were in no way reflected in the level of compliance.
Discussion
The results of this study indicate that CCM promotes growth of hair in the scalp of patients with androgenetic alopecia. Based on objective quantitative parameters of hair count as well as participants subjective evaluation and investigators qualitative assessment a gradual improvement in the status of scalp and hair was revealed.
Cessation of hair loss was reported by 80% of the CCM users within 2-8 weeks and at the end of a six month trial about 70% of participants treated with CCM had benefited from this treatment. A beneficial effect was defined as an increase in HC of at least 10% over the baseline. This uncompromising cut-off point allows discrimination between changes which are within the physiological fluctuation range of hair growth cycle[9] and the treatment derived alterations. Seasonal dependent changes in HC could not be identified in this relatively small sample size. Nevertheless, these changes were assumed to even out in this study which extended over a two year period.
An increase of up to 50% in HC was observed with an average of 17.1% for the CCM and 8.9% for the control following six months of treatment. It is important to notice that hair count measurements were taken from the peripheral zone of the bald area with estimated 30% hair loss and therefore, the maximal value that could be obtained in that location is limited to these values. Indeed, in some cases, the maximal value was reached as early as 4 months after the start of the treatment. The significant outcome of this observation is that the diameter of the bald area decreased by at least 1.6cm (twice the diameter of the measured area).
The manifestation of the changes in HC as an improvement in the global appearance of hair was somewhat less obvious. Significant global differences between CCM and control, regarding the continuation of the balding process, appeared after six months of treatment. In the CCM group, 7% demonstrated continued hair loss compared to 40% in the control group where the global appearance worsened. For the participants with progressive hair loss, cessation of the balding process is of great importance especially if treatment starts at the initial stages of balding. Global photographic analysis conducted by two independent clinicians demonstrated a gradual increase in the incidence of positive changes but these observations were not statistically significant. The 6 months treatment period appears to be too short to see global positive changes although the total HC changes were significant. Participants who continued treatment up to 8-12 months did show dramatic cosmetic improvement (data not shown). The delayed global expression of the increase in HC may be attributed to the newly growing hair type. However, the contribution of short terminal hair as well as the vellus and intermediate hair to an observable cosmetic improvement is minor.
In the assessment of efficacy of cosmetic treatment, satisfaction of the participant with the results of the treatment is of a great importance. For bald men who care about their appearance, even slowing the rate of hair loss is considered an improvement. Blinded to the treatment administered to them, the majority of CCM group felt that the treatment had a positive effect on their hair. There was complete consensus that CCM caused cessation of hair loss. Furthermore, there was unanimous agreement that the existing hair was growing at a faster rate and seemed stronger. The texture and color of the scalp and sometimes also of the hair was changed. The scalp skin which previously was dry, scaly and abraded became smooth, white and clear of abrasions and dandruff, while the hair was softer and silkier and seemed to have more body i.e. thicker.
Compared to control, the CCM was found beneficial in all the measured parameters. However, there were some participants who manifested a great improvement with the application of the vehicle itself. Several possible factors could account for this result such as the use of a mild shampoo and daily massage as well as hydration of the scalp. The latter is known to be an important factor in cosmetic skin treatments.[10]
The skin penetration promoter used in this study was hydration of the scalp prior to the application of the gel. The gel preparation does not contain any skin penetration promoters. It was shown that application of water to the treated site is an effective penetration promoter and optimal transdermal delivery is enhanced by application of the product to hydrated skin for 3 hours.[11, 12] Therefore, the purpose of using a plastic cap was to create an occlusive cover which prevents rapid dehydration and thus extends the effect of the CCM. Still, the hair counts changes were not found to be affected by the duration of occlusive application. This might be attributed to the observed 4.6± 2 hrs/day occlusive gel application which is well beyond the previously reported optimal hydration of 3 hrs. It could be that optimal results can be obtained with a shorter treatment duration, making this treatment more user friendly. The optimal length of treatment is yet to be determined.
Treatment of androgenetic alopecia with CCM showed dynamics of hair growth similar to those observed with Minoxidil.[13, 14, 15, 16,17, 18, 19] The mean increase in total HC following 6 months of CCM treatment is 14.5hair/0.5cm2which is equivalent to the Minoxidil reported increase of 120-160 hair in 5cm2, an area which is x10 larger. However, there were no reported or observed side effects in the CCM treated group while the Minoxidil had side effects of varied types and frequencies.[16,20] The CCM genetically engineered hormonal components are present in serum level concentrations, degrade rapidly, and therefore do not accumulate. No side effects were reported or observed in any of the 200 patients treated with similar composition on open wounds, where there is no skin barrier. Therefore, the negligible albeit daily quantity of CCM applied per kg body weight and the skin barrier make it even safer to use.
The beneficial effects of CCM in both wound healing and hair growth could be combined to improve the efficacy of autologous hair micrografts.
Conclusioni:
Application of CCM to the hydrated scalp skin stimulated the hair follicles. It induced revitalization of the hair as indicated by cessation of hair loss, increased rate of growth of the existing hair and finally growth of new hair.
Tables
Table I. Number of participants 48 : 30-CCM, 18-control
Range Averagea Age, years 18 – 37 28 ± 4 Duration of Hair Loss, years 0.5 – 12 4.7 ± 3.4 Initial Hair Count, number/0.5cm2 42 – 141 94 ± 23
aThere were no statistically significant differences between CCM and control. Range and average values were almost identical for both groups.
Table II. Average Change From Baseline of Total Hair Count (Area of 0.5cm2)
Vehicle Control CCM Active # hair ± SD n # hair ± SD n p value T2 6.5 ± 7.9 14 7.3 ± 11.2 29 NS T4 2.8 ± 10.2 16 11.4 ± 11.7 29 0.015 T6 7.6 ± 8.2 18 14.5 ± 13.5 30 0.033
Table III. Change of Total Hair Count as Percent of Baseline
Vehicle Control CCM Active % change ± SD n % change ± SD n p value T2 6.8 ± 7.0 14 8.6 ± 12.3 29 NS T4 4.1 ± 13.0 16 13.8 ± 14.5 29 0.027 T6 8.9 ± 9.9 18 17.1 ± 16.4 30 0.035